Effects of CYP3A4 and CYP2C9 genotype on systemic anastrozole and fulvestrant concentrations in SWOG S0226
Metadata
Authors: Delaney V Rutherford, Sarah Medley, Nicholas C Henderson, Christina L Gersch, Ted A Vandenberg, Kathy S Albain, Shaker R Dakhil, Nagendra R Tirumali, Julie R Gralow, Gabriel N Hortobagyi, Lajos Pusztai, Rita S Mehta, Daniel F Hayes, Kelley M Kidwell, N Lynn Henry, William E Barlow, James M Rae, Daniel L Hertz Journal: Pharmacogenomics Date: 2023 Aug 23 DOI: 10.2217/pgs-2023-0097 PMID: 37615099 PMCID: PMC10565537 URL: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10565537/ PDF: https://pmc.ncbi.nlm.nih.gov/articles/PMC10565537/pdf/pgs-24-665.pdf
Abstract
Objective & methods:: This study tested associations of genotype-predicted activity of CYP3A4, other pharmacogenes, SLC28A7 (rs11648166) and ALPPL2 (rs28845026) with systemic concentrations of the endocrine therapies anastrozole and fulvestrant in SWOG S0226 trial participants.
Results:: Participants in the anastrozole-only arm with low CYP3A4 activity (i.e. CYP3A4*22 carriers) had higher systemic anastrozole concentrations than patients with high CYP3A4 activity (β-coefficient = 10.03; 95% CI: 1.42, 18.6; p = 0.025). In an exploratory analysis, participants with low CYP2C9 activity had lower anastrozole concentrations and higher fulvestrant concentrations than participants with high CYP2C9 activity.
Conclusion:: Inherited genetic variation in CYP3A4 and CYP2C9 may affect concentrations of endocrine therapy and may be useful to personalize dosing and improve treatment outcomes.
Keywords: anastrozole, drug–drug interaction, fulvestrant, metabolism, pharmacogenetics, pharmacokinetics
Objective & methods:
This study tested associations of genotype-predicted activity of CYP3A4, other pharmacogenes, SLC28A7SLC28A7 (rs11648166) and ALPPL2ALPPL2 (rs28845026) with systemic concentrations of the endocrine therapies anastrozole and fulvestrant in SWOG S0226 trial participants.
Results:
Participants in the anastrozole-only arm with low CYP3A4 activity (i.e. CYP3A422CYP3A422 carriers) had higher systemic anastrozole concentrations than patients with high CYP3A4 activity (β-coefficient = 10.03; 95% CI: 1.42, 18.6; p = 0.025). In an exploratory analysis, participants with low CYP2C9 activity had lower anastrozole concentrations and higher fulvestrant concentrations than participants with high CYP2C9 activity.
Conclusion:
Inherited genetic variation in CYP3A4CYP3A4 and CYP2C9CYP2C9 may affect concentrations of endocrine therapy and may be useful to personalize dosing and improve treatment outcomes.
**Keywords:**Keywords: anastrozole, drug–drug interaction, fulvestrant, metabolism, pharmacogenetics, pharmacokinetics
Methods
Enrollment in S0226
Patients with HR+ metastatic breast cancer were eligible to enroll in the SWOG S0226 trial if they had not received prior chemotherapy, immunotherapy or endocrine therapy for metastatic disease [55]. Adjuvant chemotherapy treatment, if given, had to be completed at least 1 year prior to enrollment. Prior tamoxifen therapy was acceptable. Patients were excluded from the trial if they were receiving treatment with an anticoagulant or had another malignancy. Patients were randomly assigned 1:1 to either anastrozole 1 mg orally daily alone, or with fulvestrant 500 mg intramuscular loading dose and 250 mg at day 14, day 28 and then monthly. The S0226 protocol was amended during the study to increase the fulvestrant maintenance dose from 250 to 500 mg monthly. Treatment continued until disease progression, unacceptable toxicity, a treatment delay of 4 or more weeks or patient withdrawal.
Measurement of systemic drug concentrations
All participants on the S0226 pharmacokinetic substudy were enrolled and treated with 250 mg fulvestrant maintenance dose before the amendment increasing the dose to 500 mg. The details of sample collection and systemic drug concentration measurement have been previously reported [66]. Briefly, 5 or 10 ml blood samples were collected just prior to dosing, approximately 24 h after the last anastrozole dose and 14 days or 1 month after the last fulvestrant dose, for estimation of trough concentration on days 14 and 28 on the combination arm and at months 2, 4, 6 and 8 on both arms. Samples were processed to isolate plasma and stored at -20°C. Anastrozole and fulvestrant concentrations were measured using previously described liquid chromatography with tandem mass spectrometry assays [66].
Pharmacogene & candidate variant genotyping
Whole blood was collected pretreatment from S0226 trial participants for isolation of germline DNA for pharmacogenetic analysis. Genotyping of candidate pharmacogenes was conducted on the iPLEX ADME PGx Pro Panel by Agena Biosciences (CA, USA). The panel evaluated >150 polymorphisms in 36 genes, including CYP3A42CYP3A42, CYP3A46CYP3A46, CYP3A420CYP3A420, CYP3A422CYP3A422, CYP2C92, CYP2C93CYP2C92, CYP2C93 (or1818), CYP2C94, CYP2C95, CYP2C96, CYP2C98, CYP2C99, CYP2C910, CYP2C911, CYP2C912, CYP2C913, CYP2C915, CYP2C925*, CYP2C94, CYP2C95, CYP2C96, CYP2C98, CYP2C99, CYP2C910, CYP2C911, CYP2C912, CYP2C913, CYP2C915, CYP2C925 and CYP2C927CYP2C927. Noncarriers of any of these variants were assigned the wild-type (CYP3A41CYP3A41 or CYP2C91CYP2C91) genotype. Raw genotype calls for each polymorphism were translated into haplotypes and metabolic activity phenotypes, as previously described [88]. This translation was modeled after the Clinical Pharmacogenetics Implementation Consortium process [99], with necessary variations to accommodate genes and variants that are not included in Clinical Pharmacogenetics Implementation Consortium guidelines. For CYP3A4CYP3A4 and CYP2C9CYP2C9, alleles considered to be reduced activity included CYP3A420CYP3A420, CYP3A422CYP3A422, CYP2C92, CYP2C93, CYP2C95, CYP2C96, CYP2C98, CYP2C911CYP2C92, CYP2C93, CYP2C95, CYP2C96, CYP2C98, CYP2C911 and CYP2C912CYP2C9*12; all other alleles were considered normal activity. Each patient was characterized as a poor (PM), intermediate (IM), normal (NM) or ultra-rapid (UM) metabolizer. For CYP3A4CYP3A4 and CYP2C9CYP2C9, a PM is a patient carrying two reduced activity alleles, an IM is a patient carrying one reduced activity allele and an NM is a patient carrying no reduced activity alleles. Before analysis, patients were classified into ‘low’ and ‘high’ phenotypes for each gene by grouping phenotypes into two groups to maximize the number of patients in the smaller group (e.g., PM vs IM/NM/UM or PM/IM vs NM/UM).
In addition, genome-wide genotyping of S0226 patients was conducted using the Illumina Infinium Global Screening Array by the University of Michigan Advanced Genomics Core. Genotyping and genetic data quality control were conducted as previously described [1010]. Candidate variants in SLC28A7SLC28A7 (rs11648166) and ALPPL2ALPPL2 (rs28845026) that were previously reported to be associated with anastrozole systemic concentrations [77] were obtained from genome-wide genotyping data. The rs11648166 and rs28845026 single nucleotide variants were not incorporated onto the Illumina genotyping array used; therefore, the strongly linked variants SLC28A7SLC28A7 rs16960359 (r^2^2 = 0.99; D′ = 1.0) and ALPPL2ALPPL2 rs883013 (r^2^2 = 0.92; D′ = 0.97), respectively, were used in the analysis. There were insufficient numbers of patients in this pharmacokinetic substudy for genome-wide association testing of noncandidate variants.
Statistical analysis
Anastrozole and fulvestrant concentrations were compared between low and high metabolic phenotypic activity groups in the candidate pharmacogene analysis. The a prioria priori selected primary hypothesis was that patients with low CYP3A4 activity would have higher anastrozole concentrations. The primary analysis included anastrozole measurements at months 2, 4, 6 and 8 using a linear mixed effects model with a random intercept, a random slope for time and an unstructured variance-covariance matrix. Analyses of anastrozole concentration were adjusted for the treatment arm due to the known effect of fulvestrant on anastrozole systemic concentrations [66]. PostPost hochoc analyses were conducted stratified by treatment arm to explore whether there was an effect of metabolic activity in either arm. Exploratory pharmacogenetic analyses of all other genes with anastrozole and fulvestrant were conducted similarly to the primary analysis. Candidate variant analyses of SLC28A7SLC28A7 rs16960359 and ALPPL2ALPPL2 rs883013 assumed a dominant genetic effect comparing patients who carried at least one variant allele with patients homozygous for the wild-type allele. All analyses were conducted using two-sided α = 0.05 without multiplicity adjustment. The a prioria priori selected primary analysis of CYP3A4 metabolic activity with anastrozole concentration should be considered hypothesis-directed and all other analyses should be considered exploratory. All analyses were conducted using R statistical software.
Results
Patients, genetics & systemic drug concentrations
Of the 707 patients enrolled on S0226, 92 had anastrozole and/or fulvestrant concentrations measured at least once during treatment, 40 in the anastrozole alone arm and 52 in the anastrozole–fulvestrant combination arm. The number of patients with measured drug concentrations who also had genetic data and were included in the analyses of anastrozole was 79; for fulvestrant, 52. The median age of the patients included in the analysis was 63, 91% were Caucasian and 39% had received prior chemotherapy treatment (Table 1Table 1).
Table 1. . Clinical information for patients included in the analysis (numbers are mean [standard deviation] or n [%]).
| Characteristic | Anastrozole measurement †, n = 79 (%) | Fulvestrant measurement, n = 52 (%) |
|---|---|---|
| Age | 63.7 (9.2) | 63.8 (9.3) |
| Race | ||
| White | 72 (91) | 47 (90) |
| Black | 5 (6) | 4 (8) |
| Other/unknown | 2 (3) | 1 (2) |
| S0226 treatment arm | ||
| Anastrozole alone | 40 (51)† | 0 |
| Anastrozole–fulvestrant combination | 39 (49)† | 52 (100) |
| Prior adjuvant endocrine treatment | ||
| Tamoxifen | 31 (39) | 21 (40) |
| None | 48 (61) | 31 (60) |
| HER2 status | ||
| Positive | 3 (4) | 4 (8) |
| Negative | 62 (78) | 40 (77) |
| Missing | 14 (18) | 8 (15) |
| Anastrozole concentrations at each time point | ||
| 2 months | 72 (91) | 44 (85) |
| 4 months | 74 (94) | 48 (92) |
| 6 months | 76 (96) | 48 (92) |
| 8 months | 79 (100) | 51 (98) |
| Fulvestrant concentrations at each time point | ||
| 2 months | 44 (85) | |
| 4 months | 48 (92) | |
| 6 months | 48 (92) | |
| 8 months | 51 (98) | |
| The numbers of patients included in the analyses with low and high phenotype activity for each gene and the wild-type and variant carrier for each of the candidate polymorphisms are reported in Supplementary Table 1Supplementary Table 1. For CYP3A4CYP3A4, only the CYP3A422CYP3A422 variant was identified, and all carriers were heterozygous (CYP3A41/22CYP3A41/22) and were assigned IM phenotype and CYP3A4 low activity (n = 12 in anastrozole analysis, n = 4 in fulvestrant analysis). Variant alleles of CYP2C9CYP2C9 that were detected included CYP2C92, CYP2C93, CYP2C98, CYP2C99, CYP2C911CYP2C92, CYP2C93, CYP2C98, CYP2C99, CYP2C911 and CYP2C912CYP2C912, which were translated into PM (n = 2 anastrozole, n = 1 fulvestrant) and IM (n = 20 anastrozole, n = 12 fulvestrant) phenotypes as described in the methods section, all of whom were included in the CYP2C9 low activity group. The median systemic concentration of anastrozole was 32 ng/ml (range: 21–67) and fulvestrant was 8 ng/ml (range: 1–18). |
Association of systemic drug concentrations with pharmacogene activity or candidate variants
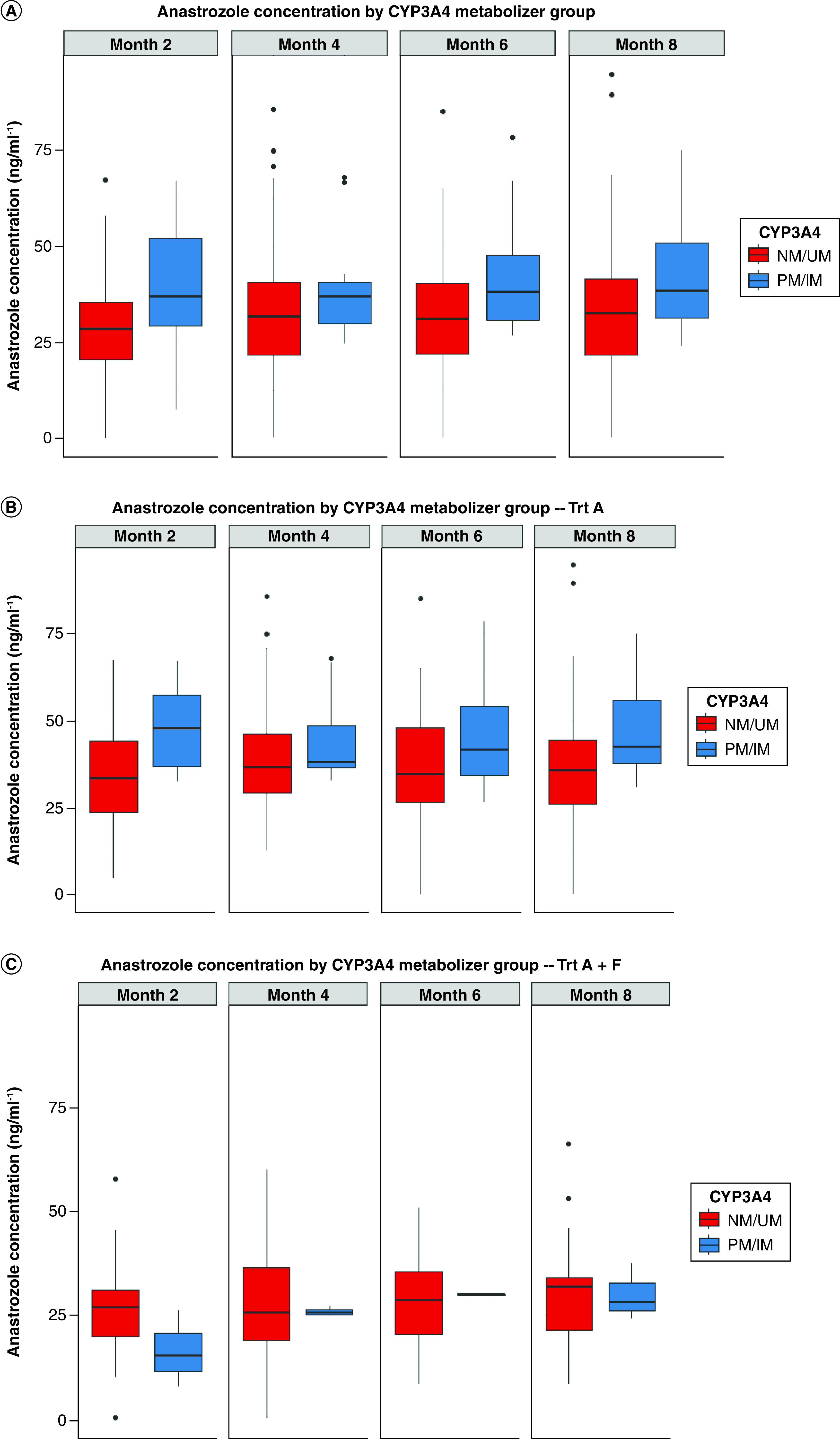
In the primary analysis, there was no difference in systemic anastrozole concentrations between low and high CYP3A4 activity phenotype groups (p = 0.13; Table 2Table 2 & Figure 1Figure 1A). However, the postpost hohoc analysis revealed the expected association of low CYP3A4 activity with higher anastrozole concentration in the anastrozole-only arm (β-coefficient = 10.03; 95% CI: 1.42, 18.6; p = 0.025; Figure 1Figure 1B). This association was not found in the anastrozole and fulvestrant combination arm (p = 0.50; Figure 1Figure 1C).
Table 2. . Association of activity phenotype with anastrozole and fulvestrant systemic concentrations.
| Gene | Comparison | Anastrozole systemic concentration† (n = 79) | Fulvestrant systemic concentration (n = 52) |
|---|---|---|---|
| β (95% CI) | p-value | ||
| CYP3A4 | PM/IM vs NM | 5.8 (91.6, 13.2) | 0.13 |
| CYP3A5 | PM vs IM/NM | 1.1 (-1.6, 13.2) | 0.77 |
| ABCB1 | PM vs IM/NM | 1.2 (-4.9, 7.3) | 0.70 |
| ABCC2 | PM/IM vs NM/UM | 1.6 (-4.2, 7.3) | 0.60 |
| SULT1A1 | PM/IM vs NM | 0.6 (-5.0, 6.3) | 0.83 |
| UGT1A1 | PM/IM vs NM | 3.1 (-2.4, 8.6) | 0.27 |
| CYP2B6 | PM/IM vs NM | -0.3 (-6.2, 5.6) | 0.93 |
| SLCO1B1 | PM/IM vs NM | 0.4 (-5.1, 5.9) | 0.89 |
| CYP2C8 | PM/IM vs NM | -3.4 (-9.6, 2.8) | 0.29 |
| CYP2C9 | PM/IM vs NM | -8.1 (-13.7, -2.5) | 0.006 |
| CYP1A1 | PM/IM vs NM | 0.7 (-6.2, 7.5) | 0.84 |
| CYP1A2 | PM/IM/NM vs UM | 2.5 (-6.6, 11.6) | 0.59 |
| UGT2B17 | PM/IM vs NM | -4.7 (-9.8, 0.4) | 0.07 |
| CYP2C19 | PM/IM vs NM/UM | -3.7 (-8.9, 1.6) | 0.18 |
| CYP2A6 | PM/IM vs NM | 0.5 (-5.9, 6.9) | 0.87 |
| VKORC1 | PM/IM vs NM | 0.8 (-4.9, 6.5) | 0.78 |
| ABCG2 | PM/IM vs NM | -4.6 (-10.5, 1.4) | 0.14 |
| UGT2B7 | PM vs IM/NM | -0.5 (-6.4, 5.5) | 0.88 |
| SLC15A2 | PM vs IM/NM | -1.2 (-7.2, 4.7) | 0.69 |
| SLC22A2 | PM/IM vs NM | -0.18 (-6.5, 6.1) | 0.96 |
| SLC22A6 | PM/IM vs NM | -5.7 (-28.9, 17.6) | 0.63 |
| SLCO1B3 | PM vs IM/NM | -4.9 (-10.4, 0.69) | 0.09 |
| TPMT | PM/IM vs NM | 0.02 (-8.4, 8.5) | 0.97 |
| CYP2E1 | IM vs NM/UM | 0.84 (-4.7, 6.4) | 0.77 |
| NAT1 | PM/IM vs NM | -2.9 (-13.8, 7.9) | 0.60 |
| NAT2 | PM vs IM/NM | 1.3 (-4.1, 6.7) | 0.63 |
| UGT2B15 | PM vs IM/NM | -5.2 (-10.9, 0.5) | 0.08 |
| SLCO2B1 | PM/IM vs NM | -2.4 (-16.0, 11.2) | 0.73 |
| SLC22A1 | PM/IM vs NM | -0.06 (-5.4, 5.3) | 0.98 |
| GSTT2B | PM/IM vs NM | -3.4 (-8.8, 2.0) | 0.22 |
| GSTT1 | PM/IM vs NM | -2.2 (-9.4, 4.9) | 0.54 |
| GSTP1 | PM/IM vs NM | 0.5 (-5.4, 6.3) | 0.87 |
| GSTM1 | PM/IM vs NM | -3.6 (-8.8, 1.6) | 0.18 |
| DPYD | PM/IM vs NM | NA | NA |
| CYP2D6 | PM/IM vs NM/UM | 1.2 (-4.1, 6.6) | 0.65 |
| COMT | PM vs IM/NM | 0.24 (-5.2, 5.7) | 0.93 |
| SLC28A7 rs16960359 | Variant carrier vs wild type | -2.1 (-4.8, 9.0) | 0.55 |
| ALPPL2 rs883013 | Variant carrier vs wild-type | 3.1 (-9.1, 2.9) | 0.31 |
Figure 1. . Systemic anastrozole concentration by CYP3A4 metabolic activity.
(A) Systemic anastrozole concentrations in all patients with measured anastrozole systemic concentration by CYP3A4 metabolic activity phenotype over time (p = 0.13). (B) Systemic anastrozole concentrations in the anastrozole-only treatment arm (p = 0.025). (C) Systemic anastrozole concentrations in the anastrozole and fulvestrant combination arm (p = 0.50). Boxplots present the median and interquartile range of anastrozole concentrations for each CYP3A4 metabolic activity phenotype group. IM: Intermediate metabolizer; NM: Normal metabolizer; PM: Poor metabolizer; UM: Ultra-rapid metabolizer.
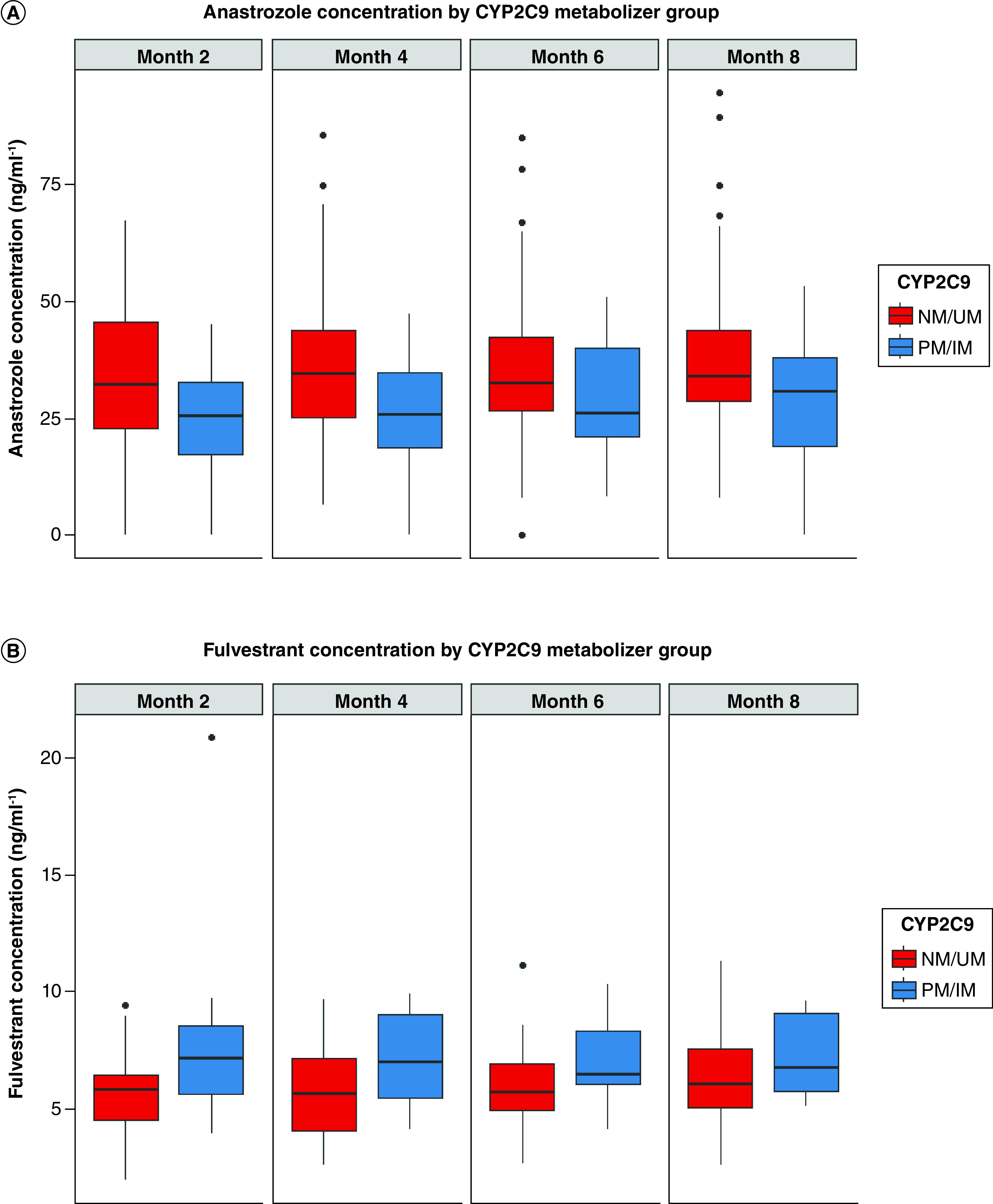
In the exploratory analyses of the remaining genes with anastrozole and fulvestrant, patients with low CYP2C9 metabolic activity had lower anastrozole (β-coefficient = -8.1; 95% CI: -13.7, -2.5; p = 0.006; Table 2Table 2 & Figure 2Figure 2A) and higher fulvestrant (β-coefficient = 1.1; 95% CI: 0.08, 2.2; p = 0.041; Table 2Table 2 & Figure 2Figure 2B) systemic concentrations than patients with high CYP2C9 activity. Metabolic phenotypic activity for other tested pharmacogenes was not associated with anastrozole or fulvestrant systemic concentrations (all p > 0.05; Table 2Table 2). Similarly, neither of the candidate polymorphisms, SLC28A7SLC28A7 rs16960359 nor ALPPL2ALPPL2 rs883013, were associated with systemic anastrozole concentration (both p > 0.05; Table 2Table 2 & Supplementary Figure 1Supplementary Figure 1).
Figure 2. . Systemic anastrozole concentration by CYP2C9 metabolic activity.
(A) Systemic anastrozole concentrations in all patients by CYP2C9 metabolic activity phenotype over time (p = 0.006). (B) Systemic fulvestrant concentrations in the anastrozole–fulvestrant combination arm (p = 0.041). The boxplots present the median and interquartile ranges of anastrozole and fulvestrant concentrations for each CYP2C9 metabolic activity phenotype group over time. IM: Intermediate metabolizer; NM: Normal metabolizer; PM: Poor metabolizer; UM: Ultra-rapid metabolizer.
Discussion
Variability in systemic concentrations of anastrozole and fulvestrant may contribute to variable treatment response and toxicity, and it may be caused by inherited genetic variation and drug–drug interactions [1111]. This study aimed to identify pharmacogenetic associations with systemic concentrations of anastrozole and fulvestrant. As hypothesized, patients with reduced CYP3A4 activity had higher concentrations of anastrozole when used alone, though this association was not seen in patients also receiving fulvestrant, which is not a standard-of-care regimen used in most patients. The authors also found evidence that lower CYP2C9 activity may be associated with lower concentrations of anastrozole and higher concentrations of fulvestrant.
The main finding supported the primary hypothesis that patients with lower CYP3A4 activity have higher steady-state systemic anastrozole concentrations. In this analysis, only the reduced activity CYP3A422CYP3A422 [1212] variant was detected, so it is not possible to investigate whether any other CYP3A4CYP3A4 variants, such as the inactive CYP3A420CYP3A420 [1313] allele, affect anastrozole metabolism. The association of reduced activity CYP3A4CYP3A4 polymorphisms with anastrozole pharmacokinetics has not been investigated, to the authors' knowledge, and prior studies have not found associations of CYP3A4CYP3A4 genotype with downstream phenotypes such as changes in bone mineral density [1414]. The authors of the present study were unable to replicate associations with anastrozole systemic concentrations for two polymorphisms (SLC28A7SLC28A7 rs16960359 and ALPPL2ALPPL2 rs883013) recently identified within a genome-wide association study, although further attempted validation in a larger cohort is needed to determine their clinical relevance [77].
Exploratory analyses of other pharmacogenes indicate that patients with lower CYP2C9 activity, primarily due to carrying the low activity CYP2C92CYP2C92 or CYP2C93CYP2C93 variants, had, surprisingly, lower anastrozole concentrations and higher fulvestrant concentrations. Low activity CYP2C9 phenotype is associated with reduced metabolism, and therefore higher systemic concentrations, of various drugs, including tamoxifen and warfarin [88,1515]. Previous studies have speculated that lower CYP2C9 activity may be associated with higher anastrozole concentrations [1616], but CYP2C9 is not known to contribute to fulvestrant metabolism [44]. The explanation for these unexpected findings is not known and may be a false association caused by the lack of correction for multiple comparison testing. However, it is also possible that patients with low CYP2C9 activity have higher fulvestrant concentrations, which caused a greater interaction between these drugs [66] and resulted in lower anastrozole concentrations, though there were insufficient numbers of patients to investigate this hypothesis. Additional studies in larger cohorts of patients will be needed to confirm the effects of CYP2C9 activity on fulvestrant concentrations and perhaps the downstream effects on anastrozole concentrations.
Although this study identified pharmacogenetic predictors of systemic concentrations of anastrozole and fulvestrant, the analyses did not investigate downstream effects on clinical outcomes, including estrogenic response, efficacy or toxicity. Prior studies have not identified evidence that systemic concentrations of anastrozole, fulvestrant or other aromatase inhibitors are associated with estrogenic suppression, efficacy or toxicity [1414,16–1816–18], although the genome-wide association study that identified the associations of SLC28A7SLC28A7 rs16960359 and ALPPL2ALPPL2 rs883013 with anastrozole systemic concentration did report higher anastrozole systemic concentration in patients with undetectable plasma estrone and estradiol [77]. Future studies will need to validate associations between systemic anti-estrogen concentrations and clinically relevant treatment outcomes before investigating and implementing personalized dosing based on inherited genetics.
This study has some limitations that should be considered. Of the 707 patients enrolled in S0226, only 92 patients with measured systemic drug concentrations and genotyped germline DNA could be included in this analysis, limiting power to detect weaker associations or associations for lower-frequency variants of activity phenotypes and precluding genome-wide association testing. Additionally, the genotyping strategy was limited to 36 pharmacogenes and two additional candidate polymorphisms. Finally, this small, exploratory study was unable to account for multiple comparisons.
Conclusion
In conclusion, patients carrying CYP3A422CYP3A422, or with low CYP3A4 activity phenotype, have higher concentrations of anastrozole compared with those with high CYP3A4 activity, when it is administered without fulvestrant. In addition, patients with low CYP2C9 activity may have higher concentrations of fulvestrant and lower concentrations of anastrozole, perhaps due to a drug–drug interaction. Future studies are needed to assess the indirect effects of CYP3A4CYP3A4 and CYP2C9CYP2C9 genetics, or the direct effects of anastrozole and fulvestrant systemic concentrations, on clinical outcomes, which could lead to the personalization of anti-estrogen dosing based on genetics and subsequently to improved treatment outcomes.
Footnotes
References
Kohler BA, Sherman RL, Howlader N et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J. Natl Cancer Inst. 107(6), djv048 (2015). DOI | PMC free article | PubMed | Google Scholar
Hamadeh IS, Patel JN, Rusin S et al. Personalizing aromatase inhibitor therapy in patients with breast cancer. Cancer Treat. Rev. 70, 47–55 (2018). DOI | PubMed | Google Scholar
Kamdem LK, Liu Y, Stearns V et al. In vitro and in vivo oxidative metabolism and glucuronidation of anastrozole. Br. J. Clin. Pharmacol. 70(6), 854–869 (2010). DOI | PMC free article | PubMed | Google Scholar
Edavana VK, Yu X, Dhakal IB et al. Sulfation of fulvestrant by human liver cytosols and recombinant SULT1A1 and SULT1E1. Pharmgenomics Pers. Med. 4, 137–145 (2011). DOI | PMC free article | PubMed | Google Scholar
Mehta RS, Barlow WE, Albain KS et al. Overall survival with fulvestrant plus anastrozole in metastatic breast cancer. N. Engl. J. Med. 380(13), 1226–1234 (2019). DOI | PMC free article | PubMed | Google Scholar
Hertz DL, Barlow WE, Kidwell KM et al. Fulvestrant decreases anastrozole drug concentrations when taken concurrently by patients with metastatic breast cancer treated on SWOG study S0226. Br. J. Clin. Pharmacol. 81(6), 1134–1141 (2016). DOI | PMC free article | PubMed | Google Scholar
Dudenkov TM, Liu D, Cairns J et al. Anastrozole aromatase inhibitor plasma drug concentration genome-wide association study: functional epistatic interaction between SLC38A7 and ALPPL2. Clin. Pharmacol. Ther. 106(1), 219–227 (2019). DOI | PMC free article | PubMed | Google Scholar
Chen Y, Marcath LA, Eliassen FM et al. Effect of genetic variability in 20 pharmacogenes on concentrations of tamoxifen and its metabolites. J. Pers. Med. 11(507), 1–12 (2021). DOI | PMC free article | PubMed | Google Scholar
Relling MV, Klein TE, Gammal RS et al. The Clinical Pharmacogenetics Implementation Consortium: 10 years later. Clin. Pharmacol. Ther. 107(1), 171–175 (2020). DOI | PMC free article | PubMed | Google Scholar
Hertz DL, Douglas JA, Kidwell KM et al. Genome-wide association study of letrozole plasma concentrations identifies non-exonic variants that may affect CYP2A6 metabolic activity. Pharmacogenet. Genomics 31(5), 116–123 (2021). DOI | PMC free article | PubMed | Google Scholar
Hertz DL, Henry NL, Rae JM. Germline genetic predictors of aromatase inhibitor concentrations, estrogen suppression and drug efficacy and toxicity in breast cancer patients. Pharmacogenomics 18(5), 481–499 (2017). DOI | PMC free article | PubMed | Google Scholar
Elens L, van Gelder T, Hesselink DA et al. CYP3A4*22: promising newly identified CYP3A4 variant allele for personalizing pharmacotherapy. Pharmacogenomics 14(1), 47–62 (2013). DOI | PubMed | Google Scholar
Westlind-Johnsson A, Hermann R, Huennemeyer A et al. Identification and characterization of CYP3A4*20, a novel rare CYP3A4 allele without functional activity. Clin. Pharmacol. Ther. 79(4), 339–349 (2006). DOI | PubMed | Google Scholar
Bojanic K, Kuna L, Bilic Curcic I et al. Representation of CYP3A4, CYP3A5 and UGT1A4 polymorphisms within Croatian breast cancer patients' population. Int. J. Environ. Res. Public Health 17(10), 3692 (2020). DOI | PMC free article | PubMed | Google Scholar
Daly AK, Rettie AE, Fowler DM et al. Pharmacogenomics of CYP2C9: functional and clinical considerations. J. Pers. Med. 8(1), 1 (2017). DOI | PMC free article | PubMed | Google Scholar
Robertson JF, Harrison M. Fulvestrant: pharmacokinetics and pharmacology. Br. J. Cancer 90(Suppl. 1), S7–S10 (2004). DOI | PMC free article | PubMed | Google Scholar
Hertz DL, Speth KA, Kidwell KM et al. Variable aromatase inhibitor plasma concentrations do not correlate with circulating estrogen concentrations in post-menopausal breast cancer patients. Breast Cancer Res. Treat. 165(3), 659–668 (2017). DOI | PMC free article | PubMed | Google Scholar
Kadakia KC, Snyder CF, Kidwell KM et al. Patient-reported outcomes and early discontinuation in aromatase inhibitor-treated postmenopausal women with early stage breast cancer. Oncologist 21(5), 539–546 (2016). DOI | PMC free article | PubMed | Google Scholar